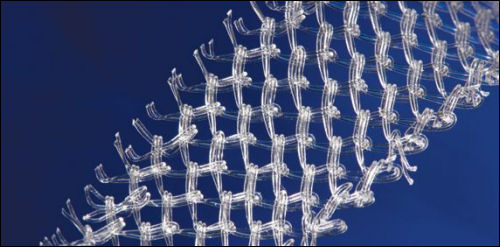
A CBC News article today reported that the US Food and Drug Administration has ordered immediate removal of surgical mesh products for prolapse to be removed from the market immediately. The companies have 10 days to submit their plans for removal of their products. The FDA indicates that companies selling the mesh have not demonstrated reasonable assurance of safety and effectiveness for the devices.
Women have been reporting mild to severe complications with mesh implants; some have been left with debilitating physical pain that has limited mobility and diminished quality of life. Lobby groups in several countries have rallied for restricted use of mesh devices and many lawsuits have been filed. Canadian women have previously launched lawsuits related to complications with mesh implantation.
Compared to more invasive surgeries, the mesh is a relatively simple procedure that has become quite commonly recommended for women suffering from pelvic health issues, including incontinence and prolapse.
For a summary of Pelvic Mesh related articles previous to this announcement, see Pelvic Mesh Risks
View the FDA Statement Here
Read the full CBC New Article ‘Stop selling transvaginal surgical mesh for pelvic organ prolapse, U.S. FDA orders’